AHL Newsletter, June 2014
For a pdf copy of AHL Newsletter, June 2014 click here
May 1, 2014, AHL User’s Guide and Fee Schedule
Effective May 1, 2014 we have updated our tests and fees. We have inactivated many of our species specific influenza tests for more “generic” labelling—whether avian, swine, equine, or canine, we’re looking for Influenza A virus.
We’ve added in many other new or modified tests over the past year: Bacterial culture/fecal culture equine, Brachyspira panel PCR, Hatchery reactor/excess mortality Salmonella, MLST molecular typing, Mycoplasma isolate prep for autogenous vaccine, Mycoplasma hyosynoviae and hyorhinis PCR, Potomac horse fever PCR, companion/other only zinc, copper and lead, Iodine in milk ICP-MS, Equid herpesvirus 1 PCR, PDCoV PCR, PEDV PCR, PEDV/TGEV/PRCV triplex PCR, Astrovirus PCR, and Bovine respiratory virus panel triplex PCR.
We have inactivated many older or less popular tests in favor of more suitable PCR tests.
Our complete test list is available in our May 1, 2014 AHL User’s Guide and Fee Schedule recently sent out to our clients. For a searchable test listing and pricing, our clients can visit us at our website at www.ahl.uoguelph.ca If you do not have client access for fees – please contact ahlinfo@uoguelph.ca , or call 519 824 4120 ext. 54320 and we will be more than happy to help you with this or anything else you might require.
Once again, we look forward to serving you!
Kudos!
Within the Animal Health Laboratory, we have many lab sections and must route samples appropriately. One client in particular, Mitchell Veterinary Services and their RVT Rhonda Kaufman, helps make this process seamless. Rhonda not only labels each specimen appropriately, and sends a legible history, she also splits the specimens according to the lab requirements.
Thank you Rhonda and Mitchell Vets for taking great care in submitting cases, your efforts are greatly appreciated!!
To freeze or not to freeze?
Josepha DeLay
Freezing and subsequent thawing has a significant negative impact on the gross and histologic appearance of tissues and organs. Tissue artefact caused by freezing can mask lesions and obscure the cause of death in postmortem examinations. Freezing artefact is especially problematic in cases with lesions involving brain, spinal cord, and gastrointestinal tract.
If an animal may be submitted for postmortem examination within 3 days of death / euthanasia, freezing should be avoided. Every effort should be made to deliver the body to the AHL as soon as possible after death, in order to preserve lesions and maximize the diagnostic value of postmortem examination. Refrigeration is preferable to freezing for storage of the body prior to delivery to the diagnostic laboratory. In situations where the interval between death and the postmortem exam will be prolonged (>3 days), freezing of the body is a viable option to prevent the equally compromising effects of autolysis on postmortem results.
AHL staff and pathologists are always available to offer advice regarding appropriate storage conditions for various clinical circumstances – please call us.
DSP Disease Surveillance Plan update
Grant Maxie
The AHL has been funded by the Ontario Ministry of Agriculture and Food - Ministry of Rural Affairs for the years 2013-2018 to develop and implement a “Disease Surveillance Plan”.
Vision:
Provide value to stakeholders through an effective and sustainable animal health network.
Objectives:
1. Develop an overall plan for the Ontario Animal Health Network (OAHN), and engage stakeholders.
2. Enhance Ontario animal disease surveillance.
3. Integrate Ontario animal disease surveillance with national surveillance.
4. Develop enhanced laboratory tools for early disease detection and surge capacity.
Progress to date includes:
- The formative stakeholder meeting was held at the Arboretum on the Guelph campus on September 10, 2013, with a theme of “Staying ahead of the curve” (the epidemic curve, that is!).
Presentations given at the meeting and feedback received from participants is posted at http://www.uoguelph.ca/omafra_partnership/en/partnershipprograms/DiseaseSurveillancePlanDSP.asp
- Appointed Dr. Maria Spinato as Deputy AHL Director to back up Dr. Grant Maxie, DSP project manager.
- Hired Andrew Vince, DVM, DVSc, Diplomate ACVP, to provide enhanced pathology input to client interactions.
- Conducted an environmental scan of client education resources.
- Hired Bruce McNab, DVM, PhD, as our part-time consulting epidemiologist.
- Began collaboration with a cross-Canada technical working group planning integration of selected provincial laboratory surveillance data into the Canadian Animal Health Surveillance Network (CAHSN).
- Hired 5 laboratory technicians to expand our technical resource and assist with development and implementation of enhanced testing, e.g., expanded molecular testing panels.
- Consulted with MAPAQ re RAIZO (Quebec Alert and Animal Health Information Network), which has a 20-year track record, and excellent results.
- Hired Melanie Barham, DVM, as Animal Health Network Coordinator to develop and implement expert networks in Ontario serving various sectors - swine, poultry, bovine, small ruminants, equine, camelids, cervids, fish, bees, companion animals.
- Continued discussions with the various Ontario veterinary organizations and commodity about how to best provide value to them through the DSP.
Antimicrobial susceptibility of mastitis pathogens isolated in the AHL mastitis section in 2012 and 2013
Durda Slavic, Beverly McEwen
There is a general lack of publicly available information regarding antimicrobial susceptibility of bacterial pathogens isolated from milk samples in Ontario. This certainly can be viewed as a disadvantage to practitioners who are using empirical therapy for mastitis cases. To provide our clients with some information regarding susceptibility patterns of common mastitis pathogens, the AHL laboratory information system was searched for years 2012 and 2013 and data are summarized in Tables 1A, 1B, 2A and 2B. The susceptibility results were available for over 2,700 isolates and data are shown as % resistant.
Data for gram-positive bacteria including CNS, S. aureus, Streptococcus dysgalactiae, Streptococcus uberis, and Enterococcus spp. are summarized in Tables 1A and 1B. Oxacillin susceptibility testing was done for coagulase-negative Staphylococcus spp. (CNS) and S. aureus only, whereas cephalosporins and trimethoprim/sulfa results are not applicable for Enterococcus spp. Enterococcus spp. must always be reported resistant to these drugs regardless of in vitro results. In general, CNS tend to be more resistant than S. aureus. Over 40% of isolates were resistant to penicillin, ampicillin, and oxacillin.
Data for gram-negative bacteria including Escherichia coli, Klebsiella spp., Serratia spp., and Pasteurella multocida are summarized in Tables 2A and 2B. Erythromycin and penicillin were tested only against P. multocida since they are not effective against other gram-negative bacteria specified below.
When extrapolating from data such as the ones shown below, one should exercise caution. It should be kept in mind that laboratory data in general are biased, being mostly associated with clinical cases and likely previous treatments. For that reason, the level of resistance in our data may be higher than in the average population. In addition, from the clinical perspective, our data are based on in vitro results. These results may or may not be reproduced in vivo. For example, both S. aureus and Serratia spp. can frequently be sensitive to some or all antimicrobials in vitro but will not respond to the treatment in vivo, particularly not in mastitis cases.
Table 1A. Susceptibility results for gram-positive bacteria isolated from milk samples in 2012. The results are expressed as % of resistant.
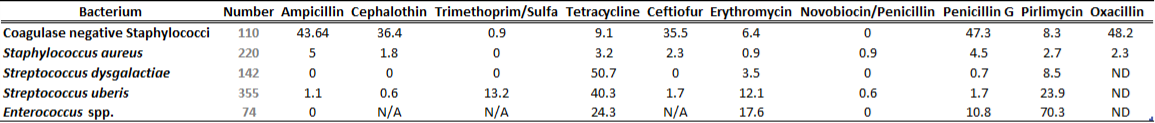
Table 1B. Susceptibility results for gram positive bacteria isolated from milk samples in 2013. The results are expressed as % of resistant.
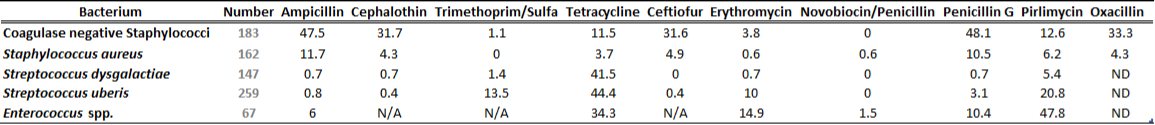
ND=not done; N/A=not applicable
Table 2A. Susceptibility results for gram-negative bacteria isolated from milk samples in 2012. The results are expressed as % of resistant.
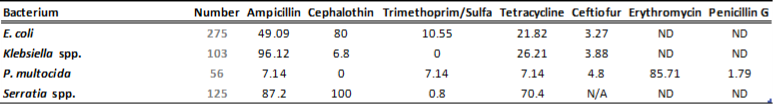
Table 2B. Susceptibility results for gram-negative bacteria isolated from milk samples in 2013. The results are expressed as % of resistant.
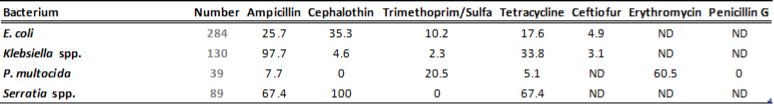
ND=not done; N/A=not applicable
RUMINANTS
Perinatal mortality secondary to goiter in goat kids
Andrew Vince, Brent Hoff
Two 1-day-old goat kids presented to the AHL had been delivered with difficulty approximately 24 h prior to presentation after difficult and prolonged labor. Noted by the clinician were large masses on the ventral aspect of each of their necks, just caudal to the jaw. The dam herself had notable hair loss and scabs on her udder.
Each goat kid was submitted in entirety for postmortem, and both had similar lesions: elongate, finger-like masses ranging from 7-10 cm in length and up to 2 cm in diameter that replaced the normal thyroid tissue (Figure 1). A tentative diagnosis of perinatal mortality secondary to hyperplastic goiter was made, and tissues were submitted for histopathology. Under the microscope, the thyroid follicular cells were columnar (rather than normal cuboidal) with large quantities of foamy-to-vacuolated cytoplasm, and frequently surrounded diminished quantities of colloid, confirming the diagnosis of hyperplastic goiter. Airways in the lungs contained occasional anucleate squamous cells, a common finding in neonates at postmortem as a result of aspiration of amniotic fluid with distress in utero.
Concurrent with postmortem assessment of the goat kids, maternal serum was submitted for assessment of iodine levels. Maternal serum contained 40.6 ppb iodine (reference range 100-400 ppb, with defined deficiency below 50 ppb), implicating a maternal iodine deficiency. Investigation on-farm found that multiple mineral sources were provided but that inappropriate mineral was provided to pregnant does; no unusual goitrogenic plants were found within feed samples.
Goiter can, in general, be attributable to one of two imbalances: a deficiency in bioavailable iodine, or a profound excess of bioavailable iodine. Deficiency can be related to either an absolute dietary deficiency in iodine, or to the concurrent consumption of antithyroid compounds (or goitrogens), such as low-level exposure to white clover, couch grass, linseed meal, and Brassica spp. (including broccoli, Brussels sprouts, cabbages, kale, turnip, rutabaga, rape, kohlrabi). Excessive iodine exposure paradoxically results in goiter as a result of serum iodide-mediated inhibition of fusion of thyroidal colloid and lysosomes, disrupting the proteolysis of colloid and release of thyroid hormones T4 and T3.
Congenital goiter is typically associated with hyperplastic goiter and the dam often shows no obvious signs of thyroid dysfunction. Gestation is often prolonged and complicated by dystocia, placental retention, and fetal weakness progressing to mortality within a few days of labor. Kids, lambs, and piglets are most susceptible, while calves and carnivores are resistant. Common findings in susceptible species are thyroid enlargement, alopecia, and myxedema; a proportion of animals may die as a result of asphyxiation secondary to tracheal compression by the enlarged thyroids.
Diagnosis of iodine deficiency disorders (IDD) has been based on morphological, histological and biochemical indices of iodine status. The evaluation of iodine status is important because of the large potential for dietary deficiency, the possibility of toxicity, and also because of the transfer of iodine to human food products, especially dairy products. While initial diagnosis of goiter-related periparturient mortality may at first seem simple, adequate clinical assessment of the underlying cause typically requires two additional steps beyond post-mortem assessment of the goat kids: serum sampling from the dam (to identify whether goiter relates to iodine deficiency or excess), and then assessment of maternal diet for points of exposure to excessive or deficient iodine levels, or to potential goitrogens. AHL
Figure 1. Ventral neck of one goat kid at postmortem. There are two roughly symmetrical 7 cm x 2 cm x 2 cm masses (representing enlarged thyroid glands) at the caudal angle of the mandible and extending over 50% of the length of the neck.

SWINE
Hemagglutinating encephalomyelitis virus (HEV) infection in piglets
Josepha DeLay, Catherine Templeton
Piglets were submitted for postmortem examination from a 1,200 gilt (P1) herd with increased piglet mortality. Piglets were clinically normal at and immediately following birth, but began to fade and waste at 5-14 days of age. No consistent gross lesions were identified in the piglets to explain the clinical signs. Histologically, nonsuppurative encephalitis was present in 5 of 7 piglets. Lesions were limited to brainstem and predominantly involved gray matter. Hemagglutinating encephalomyelitis virus (HEV) nucleic acid was identified by PCR from brain samples of all piglets, confirming the diagnosis of HEV encephalitis as the cause of mortality in this group.
HEV is a group 2 coronavirus, and is antigenically unrelated to other porcine coronaviruses such as TGEV, PEDV, and porcine respiratory coronavirus. Neurologic signs are typically seen in 4-7 day old piglets infected with HEV. In slightly older piglets (4 days-2 weeks), clinical signs of anorexia and vomiting are more common (‘vomiting and wasting disease’). Clinical disease is uncommon in older pigs.
Clinical signs in this group were limited to wasting / fading, with no evidence of vomiting or of overt neurologic deficits. Lack of sufficient anti-HEV antibody in colostrum from naïve gilts was a significant contributing factor to the outbreak in this herd. HEV infection in mixed-parity sow herds is usually self-limiting, with a 2-3 week clinical course, and infection wanes with stimulation of maternal immunity. In this P1 herd, the clinical problem lingered for 12-14 weeks but was eventually controlled using strict feedback protocols pre-breeding and at 5-7 weeks prior to farrowing to stimulate and boost gilt immunity and colostral antibody levels.
Histologic lesions support a diagnosis of HEV and the most diagnostically significant lesions are in brain, which highlights the importance of sampling brain in piglet mortality cases. Diagnosis of HEV infection is confirmed by PCR testing on nasal swabs from live pigs, or on samples from brain, tonsil, and stomach from dead animals.

Reprinted with permission, National Pork Board, USA.
HORSES
Eumycotic mycetoma in a horse
Josepha DeLay, Marc Desjardins, Graeme Doodnaught
An excisional cutaneous biopsy from a 9 year old Thoroughbred-cross mare was submitted to the AHL for histologic evaluation. The 3 by 4 cm ulcerated mass was located at the tailhead (Figure 1) and had recurred following surgical excision 2 years prior to the current surgery. Histologically, dermis and subcutis were replaced and expanded by numerous discrete 200 - 300 µm diameter aggregates of pale eosinophilic debris containing vague spherical structures and surrounded by wide collars of neutrophils, macrophages, and a few multinucleated cells (Figure 2). In biopsy sections stained with PAS and methenamine silver stains, clusters of fungal hyphae with frequent bulbous dilations were identified among the eosinophilic debris (Figure 3), confirming a diagnosis of eumycotic mycetoma.
Mycetomas develop in skin and subcutis, forming mass lesions often with draining tracts and granular debris among the discharge. Although the prefix ‘mycet-’ is synonymous with ‘myco-‘, referring to fungal etiology, mycetomas may be caused by either fungal or bacterial (Actinomyces spp.) infection. Mycetomas definitively associated with fungi are termed eumycotic mycetomas, whereas those caused by Actinomyces spp. are classified as actinomycotic mycetomas. In both conditions, microorganisms are arranged in discrete aggregates or granules giving the lesions a distinctive (and quite striking) histologic appearance.
Fungi contributing to the lesions in horses originate in soil or from plant material, and infection generally occurs by contamination of a pre-existing wound or by direct implantation of contaminated plant material, such as thorns. Lesions are typically limited to skin, and complete surgical excision can be a challenge in long-standing cases with fistulation.
 |
 |
 |
|
Figure 1. Ulcerated mycetoma at dorsal aspect of tailhead (clipped).
|
Figure 2. Mycetoma, hematoxylin and eosin stain. Multiple discrete foci of eosinophilic debris are surrounded by mixed inflammatory cells and fibrous stroma. |
Figure 3. Mycetoma, methenamine silver stain. Fungal aggregates stain black, and correspond to discrete islands of eosinophilic debris in H and E-stained sections.
|
AVIAN/FUR/EXOTIC SPECIES
A flock of 8,000 7-week-old meat turkeys, fed a ration including an anticoccidial ionophore and virginiamycin, were raised in a concrete-floored grower barn on reused litter. A sudden spike in mortality with 25 dead per day, elevated from 4 per day, caused the producer to submit birds to his poultry vet for postmortem.
At postmortem, pale caseous exudate was identified in the lumen of the small intestine and small raised white to yellow foci dotted the liver capsule but did not appear to extend into the parenchyma (Figures 1A & B).
Mortality was considered to be due to necrotic enteritis (NE) developing secondary to intestinal coccidiosis. Four days of treatment with water-soluble potassium penicillin was implemented and birds responded, however mortality recurred 5 days after the completion of the antibiotic treatment. In the experience of one of the authors (LW), it is not unusual to see recurrence of NE in turkeys following treatment. More birds were submitted for postmortem and samples of small intestine and liver were submitted for histology and fresh liver was provided for bacterial culture. Moderate numbers of Clostridium perfringens and E. coli were isolated from fresh liver.
Histologically, the intestinal lesions were typical of necrotic enteritis with foci of acute mucosal necrosis and inflammation populated by colonies of rod-shaped bacteria resembling Clostridium perfringens. In addition, numerous roundworm larvae with characteristic lateral alae were present in the lumen, penetrating into the mucosa, in association with the foci of acute mucosal necrosis and inflammation (Figure 2). No coccidial organisms were seen. In the liver, foci of granulomatous inflammation, often near portal triads, contained central cores of caseous exudate and roundworm larvae (Figure 3).
An uncommon cause of necrotic enteritis in a flock of 7-week-old Ontario meat turkeys
Marina Brash, Margaret Stalker, Lloyd Weber
The histologic diagnoses of necrotic enteritis induced by a heavy burden of intestinal Ascaridia dissimilis roundworm larvae and multinodular parasitic hepatitis secondary to roundworm larval migration were surprising, but explained why the necrotic enteritis recurred shortly after the completion of the antibiotic treatment. Anthelmintic treatment of the previous 2 flocks of turkeys raised in this grower barn had not been carried out, but the roundworm burden had been continually assessed through the routine checking of daily mortality and was negligible.
The life cycle of A. dissimilis was originally thought to be similar to Ascaridia galli, the chicken ascarid with only intra-intestinal stages. However, hepatic foci in turkey livers emerged as an issue in US processing plants in the late 1980s causing the slowing of processing lines and increased liver condemnations. From the field, there were reports of high mortality from necrotic enteritis associated with the presence of large numbers of intestinal roundworm larvae and also of reduced market weights at processing. At that time, turkey ascarid infection studies were carried out and confirmed the ability of A. dissimilis larvae to cause liver lesions. If poults are challenged from 1 day of age, liver lesions can develop within 7 days with the route of migration via the portal circulation.
Roundworm eggs are extremely resistant to environmental influences, so if turkeys are being raised on reused litter, routine deworming is highly recommended. With heavy roundworm infections, the maturation of the fourth-stage A. dissimilis into adults can be delayed, so the choice of parasiticide is also important as certain anthelmintics target the adults but are not larvicidal and it is the larvae that are tissue invasive as part of their normal life cycle.
|
|
|
 |
|
|
Figure 1A: Caseous exudate (yellow arrow) was identified in the lumen of the small intestine. |
Figure 1B: Small raised white to yellow foci dotted the liver capsule. |
Figure 2: Focus of small intestinal mucosal necrosis and inflammation containing colonies of bacteria resembling Clostridium perfringens and multiple ascarid larvae. 40X H & E |
Figure 3: Hepatic periportal multinodular inflammation with central cores of caseous exudate and roundworm larvae surrounded by macrophages, multinucleated giant cells with an outer layer of plasma cells, lymphocytes and granulocytes. 100X H & E |
COMPANION ANIMALS
A 6-year-old male German Shepherd dog died unexpectedly and the body was submitted to the AHL for postmortem examination. The dog had been alone in a home at the time of death, and was found in the proximity of the propane furnace used to heat the home. There was no indication of distress prior to death. The dog had been healthy prior to this time. The owners were concerned about carbon monoxide (CO) poisoning as the cause of death, due to recent mechanical issues with the furnace.
Carbon monoxide poisoning in a dog
Josepha DeLay
No significant gross lesions were identified during autopsy. Heart blood was collected in EDTA and heparin for CO analysis at an external laboratory. Blood analysis by gas chromatography / mass spectrometry (GC/MS) identified 66% saturation of hemoglobin by CO (carboxy-hemoglobin, COHb), confirming CO toxicosis as the cause of the dog’s death. In humans, normal COHb levels in blood can reportedly reach 4% (8% in smokers). Clinical signs are identified in humans with COHb levels over 10%, and levels greater than 40% generally result in death.
CO binds to hemoglobin with much greater affinity (>200X) than does oxygen. Resulting displacement of oxygen from hemoglobin and defective release of residual bound oxygen leads to tissue hypoxia. Neurologic and cardiorespiratory signs may be evident in animals with CO poisoning. No specific gross or histologic lesions are identified in acute fatalities due to carbon monoxide poisoning, and definitive diagnosis relies on determination of COHb levels in blood. Dogs with sublethal CO poisoning may demonstrate neuronal necrosis in anatomically specific sites in brain (globus pallidus, substantia nigra, cerebellum, hippocampus), or a more delayed form of toxicity associated with myelin degeneration in deep white matter tracts in brain. Both tissue hypoxia and hypotension likely contribute to these lesions.
Histologic lesions in this dog were limited to a few foci of acute myocardial necrosis, compatible with terminal myocardial hypoxia due to CO poisoning.
Cutaneous smooth muscle tumors in 2 ferrets
Andrew Vince, Emily Martin, Adriana Pastor, Tony van Dreumel
Cutaneous smooth muscle tumors are rare in ferrets, but have been reported in the literature as having arisen from the arrector pili muscle of the hair follicles of the skin (termed piloleiomyomas or piloleiomyosarcomas). Piloleiomyosarcoma has been designated as malignant based on nuclear pleomorphism but typically is well-demarcated and amenable to surgical excision. A recurrent variant has been described as “multiple progressive piloleiomyoma”.
Recently, AHL pathologists have examined 2 similar tumors in ferrets. A mature male neutered ferret had a focal roughly 1 cm diameter nodule above one of its eyes. Histologically, it was a well-circumscribed and expansile dermal mass composed of large strap-shaped cells with fibrillar cytoplasm, extensive anisokaryosis and binucleation, but with a low mitotic index. This mass was closely apposed to an arrector pili muscle within the dermis. The second animal was submitted through the Department of Pathobiology, and was seen in consultation. This mass was 1.5 cm diameter, 2 cm from the base of the tail, and had similar cytologic features as the first but with 1-2 mitotic figures per 400X field. For both of these tumors, immunohistochemistry was performed and demonstrated strong cytoplasmic staining for vimentin and both smooth-muscle and pan-muscle actin, a pattern consistent with a smooth muscle pattern of differentiation.
The descriptive terminology for such tumors is contentious. The well-circumscribed phenotype of these masses suggests a benign biological growth habit which, in spite of nuclear pleomorphism, would make the terms leiomyoma or piloleiomyoma most appropriate; however, the pleomorphism present in each, coupled with the mitotic index in the second mass, compels some pathologists to diagnose these as sarcomas. These were both recent submissions, and follow-up is ongoing to determine whether these are individual single benign tumors, or whether these animals might have a predisposition to multiple such masses. The fact that these masses were individual and localized suggests that these are likely individual masses and unlikely to represent the syndrome described as multiple progressive piloleiomyomas, in which the lesions formed cord-like skin plaques.
The current literature strongly suggests that such solitary masses are likely cured by complete excision regardless of pleomorphism and mitotic index, making leiomyoma or piloleiomyoma a more appropriate designation for similar well-defined dermal tumors.


