Crenosoma vulpis in dogs
Jacob Avula, Tim Pasma
Animal Health Laboratory, University of Guelph, Guelph, ON.
AHL Newsletter 2024;28(1):32.
Crenosoma vulpis, commonly known as the fox lungworm, inhabits the respiratory system (bronchi/bronchioles) of various canids, including domestic dogs. In Canada, this infection is relatively common in Atlantic region. To date, AHL has recorded four cases in dogs.
Adult female worms are ovoviviparous. The eggs are coughed up and swallowed, and the larvae hatch in the feces. These first stage larvae in the feces are eaten by terrestrial snails and slugs (intermediate hosts) and develop to infective third stage. When the definitive host ingests these snails, the larvae migrate to lungs, settle in bronchi, and mature. The pre-patent period is approximately 3 weeks.
Canine crenosomosis is usually characterized by bronchitis with a chronic dry cough and retching. Heavy parasitic burdens may induce mucopurulent discharge in the airways and the cough becomes productive. This may sometimes lead to a misdiagnosis of the condition as allergic respiratory disease.
The Baermann method of larval recovery remains the gold standard for the diagnosis of the infections caused by C. vulpis and also other lungworm infections, by taking advantage of the positive hydro/thermo-tropism exhibited by the live first stage larvae. For accurate identification of larvae, a thorough morphological and morphometric analysis must be done. The larvae measure 240-310 um and have a bluntly conical head. The tail tapers noticeably to a point with a slight deflection just before the tip (Fig.1).
Although the Baermann examination is recommended for diagnosing crenosomosis and other lungworm infections, this method has inherent limitations: it is relatively time-consuming (i.e., 12-24 hrs). False negative results can occur due to prepatent infections. Larvae are shed intermittently, a pattern typical of metastrongyloids, thus warranting repeated examinations (e.g., sample collected for three consecutive days). The sample must preferably be collected fresh (per rectum), as environmentally collected fecal samples can very quickly become contaminated with free-living nematodes, complicating the diagnosis.
When pulmonary parasitism is suspected, the veterinary clinician may also consider ordering fecal floatation/sedimentation in addition to Baermannisation, as Paragonimus and Eucoleus may also be involved, and they discharge only eggs in feces. An adequate amount of sample (~10 grams) must be sent to cover both tests.
At the AHL Parasitology lab section, a time-series observation was made on Crenosoma L1 recovery using the Baermann apparatus at different time points after storing the positive sample (moderate to heavy infection) in the refrigerator. The sample was set up by Baermann on days 3, 8, 14, and 17 after collection, and larvae were successfully recovered. However, in cases involving light infections where very few larvae are shed, the L1 recovery pattern may differ. AHL
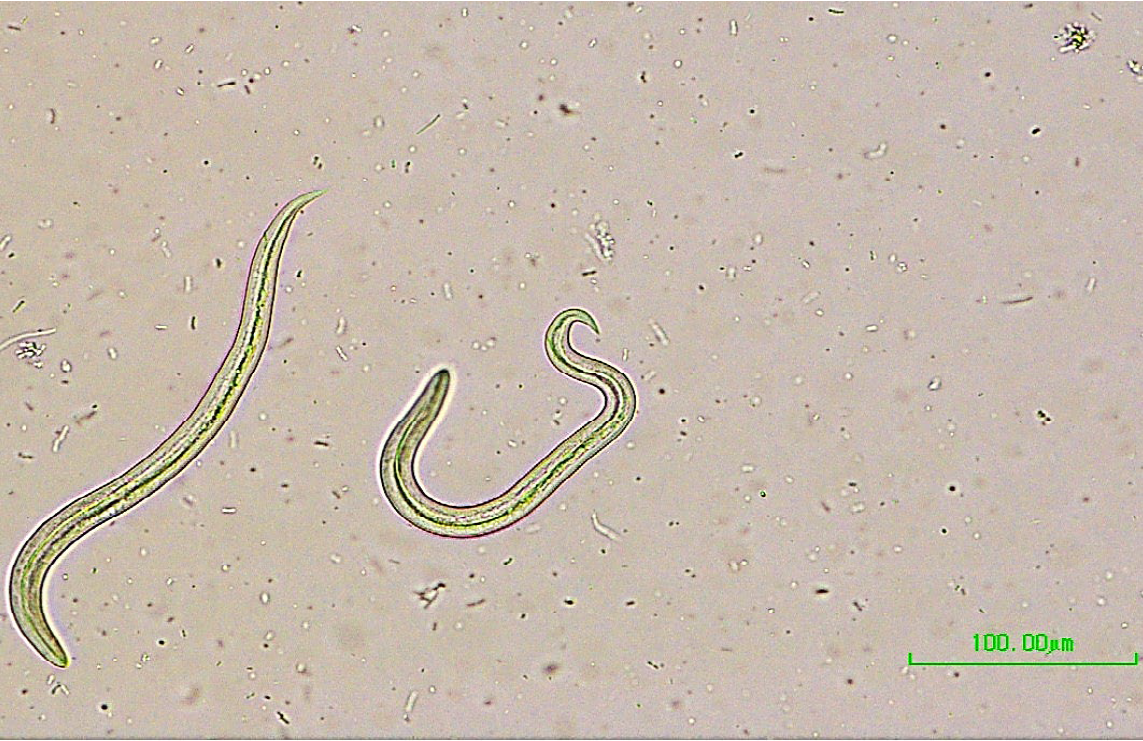
Figure 1. Crenosoma vulpis first stage larvae.
References
1. Bowman DD, Little SE. Canine pulmonary helminths: Recommendations from the Companion Animal Parasite Council. Today's Veterinary Practice May/June 2014.
2. Traversa D, Di Cesare A, Conboy G. Canine and feline cardiopulmonary parasitic nematodes in Europe: Emerging and underestimated. Parasites & Vectors 2010;3(1):62.
3. Bihr TP. Crenosoma vulpis and the domestic dog: A study of prevalence on Prince Edward Island and of new diagnostic approaches. A Thesis Submitted to the Graduate Faculty in Partial Fulfilment of the Requirements of the Degree of Master of Science in the Department of Pathology and Microbiology Faculty of Veterinary Medicine University of Prince Edward Island, Charlottetown PEI, August 1998.




