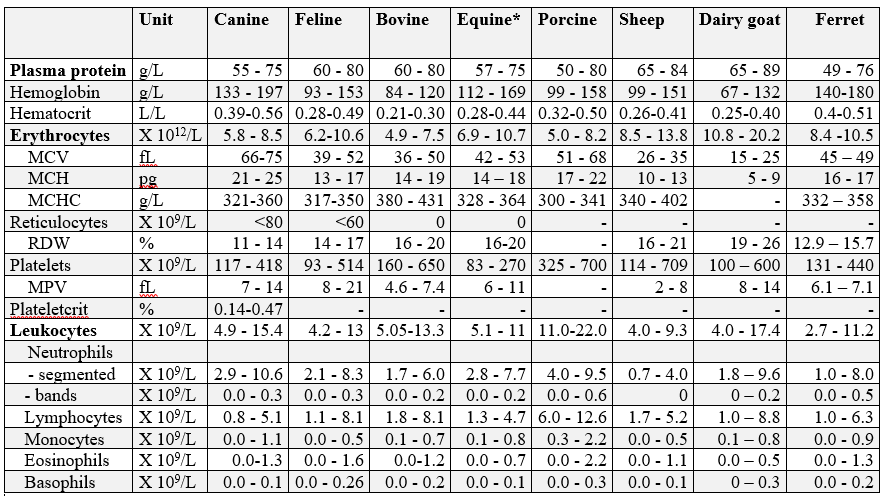
Hematology reference intervals
Details pertinent to reference interval determination can be provided by the AHL Clinical Pathology Technical Supervisor.
*Note: Horses in training may have significantly higher hemoglobin, hematocrit, and RBC parameters. A study of 419 clinically well Standardbreds, Thoroughbreds and Quarter Horses in training resulted in the following reference intervals: hemoglobin 127-188 g/L, hematocrit 0.38-0.55 L/L, and RBC 8.0-11.8 X10 12/L (central 95% intervals).
Hematology reference intervals (Rls), Advia 2120
Reference intervals were calculated using Analyse-it software (www.analyse-it.com) and parametric or non-parametric analysis.
Canine: n = 86, clinically healthy, fasted adult dogs of various breeds.
Plateletcrit n = 103 dogs with unremarkable automated CBC results, and no evidence of platelet clumping or other hematological changes on peripheral blood smear examination.
Feline: n = 40, clinically healthy, fasted adult cats of various breeds.
Bovine: n = 99, clinically healthy cows, 50% first lactation, all 30-150 days in milk.
Equine: n = 91, clinically healthy Standardbred horses.
Note: horses in training may have significantly higher hemoglobin, hematocrit, and RBC parameters. An additional study of 419 clinically healthy Standardbreds, Thoroughbreds, and Quarter Horses in training resulted in the following: hemoglobin 127-188 g/L, hematocrit 0.38-0.55 L/L, and erythrocytes 8.0-11.8 X 1012/L.
Porcine: n = 40, clinically healthy, mature sows.
Ovine: n = 63, clinically healthy sheep, various ages.
Caprine: n =37, clinically healthy dairy goats of Alpine, Toggenberg, Saanen, and Nubian breeds.
Ferrets: Erythrocyte reference intervals are represented by the minimum and maximum values for a group of 9 healthy ferrets. Leukocyte reference intervals are based on 69 clinically healthy ferrets of various ages.
Biochemistry reference intervals (RIs), cobas 6000 c501:
Canine: n = 86, clinically healthy, fasted adult dogs of various.
Feline: n = 40, clinically healthy, fasted adult cats of various breeds.
Bovine: n = 60, clinically healthy cows, 50% first lactation, all 30-150 days in milk, from 10 Ontario dairy farms.
Bovine metabolic profiles: See AHL LabNote 4, Nutritional and metabolic profile testing of dairy cows, updated March 2015.
Equine: n = 102, clinically healthy, mature horses of various breeds. Serum amyloid A reference intervals were determined from 50 clinically healthy horses from 25 farms. Serum iron determined from 49 clinically healthy horses.
Porcine: n = 40, clinically healthy, mature sows.
Ovine: n = 47, clinically healthy sheep, various ages.
Caprine: n = 37, clinically healthy dairy goats representing Alpine, Toggenberg, Saanen, and Nubian breeds.
Ferrets: n = 69, clinically healthy ferrets, various ages.
Coagulation:
Canine: n = 22, feline n = 51, equine n = 52, bovine n = 28 clinically healthy adult animals of various breeds.
Insulin:
Equine: n = 39 clinically healthy fasted (no grain) adult horses of various breeds, results compared to those of Michigan State University and Cornell diagnostic laboratories, sites using the same methodology.
Canine: n = 33 fasted samples from clinically healthy adult dogs of various breeds, results compared to those of Michigan State University and Cornell diagnostic laboratories, sites using the same methodology.
Feline: n = 42 fasted samples from clinically healthy adult cats of various breeds, results compared to those of Michigan State University and Cornell diagnostic laboratories, sites using the same methodology.
Free T4: fT4 by equilibrium dialysis, reference intervals data generated at the AHL, canine reference interval determined in consultation with the OFA.
ACTH:
Equine: n = 44 clinically healthy adult horses of various breeds, results compared to those of Michigan State University and Cornell diagnostic laboratories, sites using the same methodology.
Canine: n = 11 clinically healthy adult dogs of various breeds, results compared to those of Michigan State University and Cornell diagnostic laboratories, sites using the same methodology.
Protein electrophoresis: canine n = 13, feline n = 32, equine n = 15, clinically healthy adult animals
Fluid Cytology:
O’Brien PJ, Lumsden JH. The cytological examination of body cavity fluids. Sem Vet Med Surg (Small Animal) 1988;3:140-156.
Cowell and Tyler Diagnostic Cytology and Hematology of the Horse 2nd ed., Chapter 9 Peritoneal Fluid p.130.
Cowell and Tyler Diagnostic Cytology and Hematology of the Dog and Cat 4th ed., Chapter 12, Synovial Fluid Analysis p. 201.
Cowell and Tyler Diagnostic Cytology and Hematology of the Horse 2nd ed., Chapter 10, Synovial Fluid p.166.
CSF:
Jamieson EM. A study of cerebrospinal fluid in dogs with central nervous system disease. Thesis. University of Guelph, 1992.
Rand JS, Parent J, Jacobs R, et al. Reference intervals for feline cerebrospinal fluid: cell counts and cytologic features. Am J Vet Res 1990;1:1044-1048.
Cowell and Tyler Diagnostic Cytology and Hematology of the Horse 2nd ed., Chapter 11, Cerebrospinal Fluid, p.174.




