Understanding Your Enemy: Disrupting Cancer Communication as a Key Step in the Fight Against Breast Cancer
How did Harry Potter defeat Voldemort? It may have taken seven books, but each time Harry came one step closer, by discovering — and exploiting — Voldemort’s weakness.
This is the same approach that Dr. Nina Jones, recent PhD graduate Dr. Hayley Lau, and current PhD candidate Hayley Smith from the Department of Molecular and Cellular Biology took against a different kind of enemy: breast cancer. In collaboration with Dr. Jasmin Lalonde’s lab, the team aimed to investigate and exploit the cellular communication pathways in triple-negative breast cancer (TNBC), a highly aggressive form of breast cancer.
According to the Canadian Cancer Society, cancer remains the leading cause of death in Canada, with breast cancer being the most common cancer among women (excluding non-melanoma skin cancers), accounting for 25% of all new cancer cases in women.
Just like Voldemort had his horcruxes, TNBC may also have a weakness that can be exploited: a protein known as ShcD. ShcD is an “adaptor” protein that binds and activates other proteins, playing an essential role in a number of cell signaling pathways. It is also highly expressed in some cancers, making it a “protein of interest” when it comes to understanding what drives metastasis.
During her postdoctoral studies, Dr. Nina Jones herself took the first step in understanding this important protein, becoming the first researcher to characterize ShcD. Today, it remains a major focus of her research group at the University of Guelph — particularly when it comes to understanding its role in development and different types of disease.
"Bioinformatic screens show that ShcD is among the most upregulated genes in TNBC. We wanted to see whether ShcD might be promoting invasion in this particularly aggressive cancer subtype,” says Smith, recounting how the lab’s current study on ShcD and TNBC began.
Using a wide array of molecular and cellular approaches, the team discovered that ShcD promotes the invasiveness of TNBC by boosting a key signaling pathway linked to cell proliferation, known as EGFR signaling.
“It’s been shown that elevated EGFR signaling can increase the metastatic potential of breast cancer cells, but therapies targeting EGFR have shown limited efficacy against TNBC in clinical settings. We’ve identified ShcD as a novel driver of altered EGFR signaling in this context," explains Smith.
In fact, the team showed that interaction between ShcD and EGFR can be blocked if ShcD is inhibited by a drug. This, in turn, reduces the invasiveness of TNBC cells, presenting ShcD as a viable therapeutic target in TNBC.
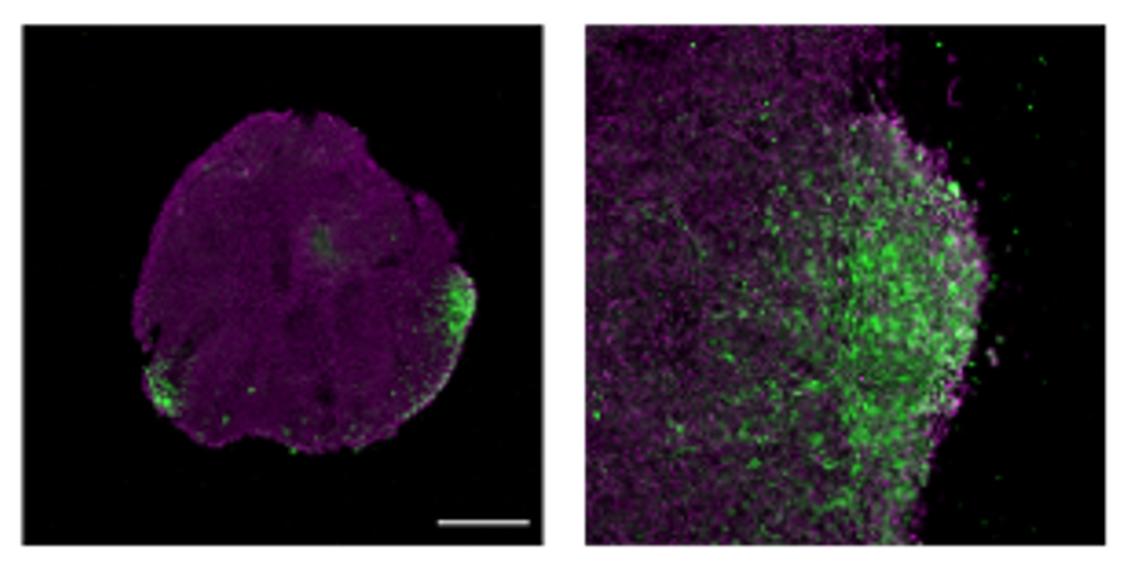
After demonstrating ShcD’s contribution to invasion by studying breast cancer cells in vitro, the team wanted to validate these findings in a model which better emulates physiological conditions in vivo. Specifically, they wanted to see if ShcD-expressing TNBC cells are also more invasive in a more complex model of biological tissue.
Here, just like Harry Potter often did, the Jones Lab also got a little help from their friends.
Enter the Lalonde lab and post-doctoral scholar Dr. Begüm Alural. Using stem cells programmed to differentiate into brain cells, the team was able to validate their in vitro findings in small brain “organoids” grown in the lab. Once again, they showed that overexpression of ShcD in breast cancer cells does, in fact, lead to greater infiltration into the brain tissue.
“These 3D ‘mini brains’ allows us to better mimic in vivo invasion. They complement our 2D in vitro studies, providing a more realistic model which can help us better understand metastatic cancers and identify more targeted treatments,” says Smith.
While fully defeating TNBC may still be a long way off, the study brings us an important step closer to not only understanding how this destructive disease operates but also identifying a new potential strategy to fight back.
Read the full study in Molecular Oncology.
Read about other CBS Research Highlights.